
hLife Journal. hLife is committed to promoting the integration and development of basic research and clinical applications. 
Clinical data show that the prevalence of metabolic syndrome (MetS) in our country is 33.9%, and the risk of cardiovascular and cerebrovascular diseases in MetS patients is 3 times higher than that of healthy people. MetS is mainly characterized by metabolic abnormalities such as insulin resistance, obesity, and hypertension, and its core pathogenesis is closely related to the interaction of chronic low-grade inflammation and immunometabolic imbalance. Recent studies have found that neutrophils, as the "pioneer immune cells" of inflammatory responses, play a key role in the development of MetS through metabolic reprogramming and functional remodeling.
Recently, Lai Guan Ng's team from Shanghai Immune Therapy Institute published a review article titled "Neutrophils: Key players in the metabolic syndrome puzzle" (Fig. 1) in the journal hLife, which systematically sorted out the effects of metabolic syndrome-induced systemic and adipose tissue-specific metabolic changes on neutrophils and their functions. and demonstrates the promise of treating metabolic syndrome by targeting neutrophils.

Figure 1 Title of the paper and author information
Basic properties of neutrophils and their metabolic mechanisms
As the most abundant white blood cell in the blood, neutrophils have unique bidirectional regulatory properties in their immune function. Traditional theory regards its migration process as a "one-way journey" from blood to tissue, but recent studies have revealed that it has the ability to reverse transendothelial reentry into blood circulation, and this dynamic migration pattern is closely related to its functional heterogeneity. Neutrophils of different phenotypes can not only build an immune defense network by releasing cytokines, but also may cause distal organ damage or systemic immunosuppression due to phenotypic polarization, Neutrophil plays a central role in acute inflammation, and its specific mechanism of action in chronic inflammation has also become a research hotspot.
Metabolic plasticity is a key feature of neutrophils to adapt to complex physiological and pathological environments. The traditional view is that it only relies on glycolysis for energy supply, but multi-omics studies have confirmed that its energy metabolism network has significant adaptability: glucose meets energy demand and effector molecule synthesis through glycolysis and pentose phosphate pathways, while fatty acid oxidation serves as energy supplementation in stress states. This metabolic flexibility is regulated by the local microenvironment, which can even induce functional reprogramming, indicating a deep association with chronic inflammatory diseases and tumor immune escape.
The developmental diversity and functional complexity of neutrophils continue to revolutionize traditional perception. Neutrophils differentiated from myelomyeloid-mononuclear progenitor cells formed subpopulations with different phenotypic functions during development, and their effect characteristics were not only shaped by local stimulus signals, but also related to the recruitment mechanism of specific subpopulations. As an innate immune baroreceptor, NLRP3 inflammasomes not only regulate the secretion of pro-inflammatory cytokines, but also affect the immune response by mediating pyroptosis. This dynamic regulatory network from development to function provides a new dimension for understanding the mechanism of neutrophils in the maintenance of immune homeostasis and the development of diseases.
Pathological mechanism and functional remodeling of neutrophils in metabolic syndrome
Neutrophils play a central role in the pathological process of MetS, and their number and function are closely related to disease characteristics. As the primary responder to metabolic inflammation, the neutrophil count in the blood circulation of MetS patients is significantly elevated, which is not only related to hyperglycemia-induced myeloid hyperhematopoiesis, but also positively correlated with metabolic indicators such as plasma glucose and triglycerides, and its sensitivity as an inflammatory marker is even better than that of traditional neutrophil-lymphocyte ratio. It is worth noting that a high-fat diet can promote granulocyte progenitor cell expansion through innate myeloid cell reprogramming, and at the same time drive senescent neutrophil liver infiltration through the CXCR4/CXCR2 axis, thereby exacerbating insulin resistance and obesity by remodeling the visceral fat immune microenvironment. The hyperglycemic environment drives the pathological activation of neutrophils through multiple mechanisms: the accumulation of glycosylation end products (AGEs) triggers oxidative stress, and the secretion of S100A8 protein stimulates the release of bone marrow hematopoietic factors to form a vicious circle, which ultimately leads to excessive neutrophil production. At the metabolic level, hyperglycemia forces an adaptive shift in neutrophil metabolism patterns - the immature stage relies on mitochondrial oxidative phosphorylation, and after maturation, it turns to glycolysis, while the abnormal activation of the pentose phosphate pathway (PPP) provides energy for NETosis under pathological conditions, and the over-released extracellular trapping nets (NETs) exacerbate tissue damage. The upregulation of neutrophil CD11b expression in hyperlipidemia further amplifies the inflammatory effect, and this abnormal metabolic-immune interaction ultimately leads to type 2 diabetes and cardiovascular complications. On the one hand, neutrophil phagocytic function and reactive oxygen species (ROS) production in diabetic patients are impaired by abnormal regulation of glycolytic enzyme insulin. On the other hand, insulin replacement therapy can directly reverse these functional deficits, highlighting the regulatory effect of metabolic interventions on immune cells. The latest evidence suggests that the overnutrition of MetS may affect the output of neutrophils' immune effects through multidimensional remodeling of the internal metabolic network of neutrophils at rest (Figure 2).
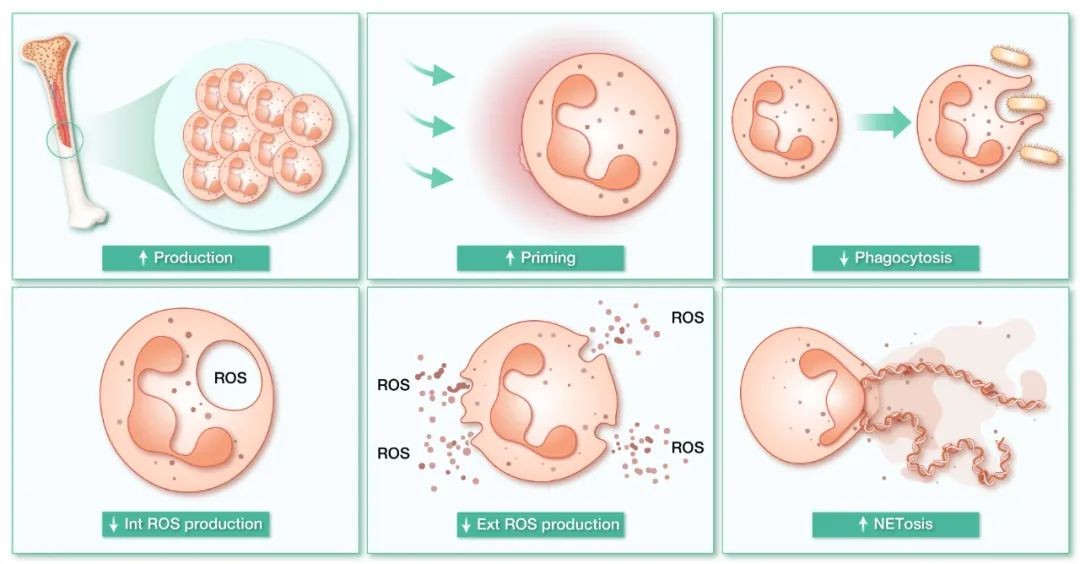
Fig. 2 Regulatory mechanism of metabolic syndrome on neutrophil effector function
Mechanism of interaction between neutrophils and adipose tissue and metabolic regulation
As a metabolic disorder caused by excess energy, the pathological core of obesity lies in abnormal expansion and dysfunction of adipose tissue (AT). Due to its anatomical location close to metabolic regulation hub organs, its excessive accumulation can exacerbate systemic metabolic disorders through abnormal endocrine function: the secretion of pro-inflammatory factors such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in AT in obese individuals increases, while the level of adiponectin, which has insulin sensitizing effects, decreases, and this adipokine imbalance is directly involved in glucose intolerance and insulin resistance. At the same time, AT will adapt lipid storage through adipocyte hypertrophy and proliferation to cope with overnutrition, but rapid expansion is often accompanied by angiogenesis lag, leading to tissue hypoxia, cell death, and waterfall release of pro-inflammatory factors, forming a chronic low-grade inflammatory microenvironment (Figure 3). Neutrophils play a key mediating role in this pathological process, and their interaction with adipocytes constitutes a vicious cycle of obesity-related metabolic disorders. In the early stage, neutrophils are recruited to AT and destroy key molecules of the insulin signaling pathway by releasing elastase, while promoting macrophage infiltration to exacerbate inflammation. Free fatty acids produced by adipocyte lipolysis enhance neutrophil-macrophage crosstalk through leukotriene B4-dependent mechanisms. Notably, there are species-specific differences in neutrophil-mediated AT inflammation: mouse models show non-NLRP3-dependent IL-1β upregulation, while human studies confirm that NLRP3 inflammasome activation is directly involved in AT fibrosis progression. The complexity of this metabolic-immune interaction network suggests that targeted neutrophil metabolic reprogramming may be a new strategy for improving obesity-related metabolic syndrome.
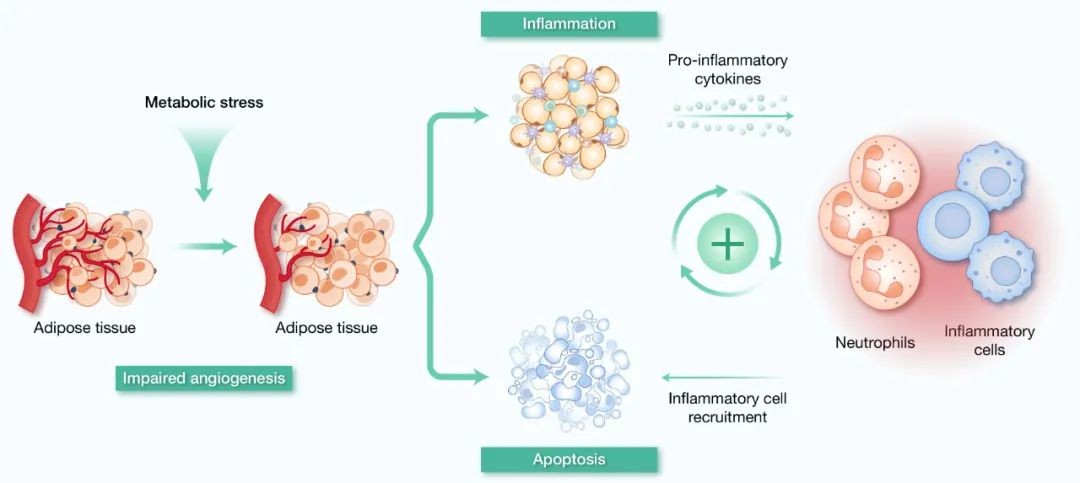
Fig.3 Pathological mechanisms of adipose tissue inflammation and immune cell recruitment in metabolic syndrome
Metabolic syndrome Pathological effects of immunometabolic disorders
As a core complication of metabolic syndrome, the pathological mechanism of type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD) is closely related to abnormal neutrophil function. The hyperglycemic environment depletes glycolysis-dependent reactive oxygen species (ROS) through the polyol/hexosamine pathway, while hyperosmolar stress inhibits mitochondrial ATP synthesis, resulting in impaired neutrophil phagocytosis and metabolic homeostasis imbalance. Although mitochondrial dysfunction has been shown to be involved in the formation of insulin resistance, its direct mechanism of action in neutrophil-mediated T2DM vascular lesions still needs to be deeply elucidated. It is worth noting that the vascular endothelial barrier undergoes inflammatory infiltration under the stimulation of hyperglycemia-induced glycosylation end products (AGEs), while neutrophil extracellular trapping networks (NETs) formed by abnormal expression of neutrophil surface receptors activate NLRP3 inflammasomes through a peptidylarginine deiminase 4 (PAD4)-dependent mechanism, forming a positive feedback loop that exacerbates vascular plaque instability and prethrombotic state.
Current research faces two major bottlenecks: differences between animal models and human mechanisms and heterogeneity of clinical samples, but key progress has been made in the field of translational medicine. Inhibitors of PAD4, the core enzyme of NETosis, entered phase II clinical trials, and mitochondria-targeted antioxidants showed therapeutic potential in regulating NLRP3 inflammasomes, while calcium signaling modulators improved metabolic-immune axis disorders by restoring neutrophil phagocytosis. These breakthroughs provide new tools to decipher the "double-edged sword" effect of neutrophils in metabolic syndrome-related diseases.
Future research needs to achieve three major leaps: analyzing the heterogeneity of neutrophil subsets through single-cell sequencing technology, and accurately simulating the pathological microenvironment in combination with vascular organoid models; build an artificial intelligence-driven multi-omics integration platform to establish a dynamic prediction model for disease progression; Breaking through the technical barriers of humanized animal models, the core position of neutrophil mitochondrial regulatory networks in the metabolic-immune-vascular interaction axis is systematically analyzed. These innovations will promote the transformation of clinical intervention strategies from single-target to multi-dimensional regulation, opening up a new path of precision medicine for the prevention and treatment of metabolic syndrome complications.
Summary and outlook
As the core pathological basis of type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD), metabolic syndrome faces the dual challenges of long-term health damage and economic burden. Studies have confirmed that chronic inflammation of adipose tissue (AT) is a key early driver of metabolic syndrome, often preceding obesity and T2DM. As the core regulatory node of the inflammatory network, neutrophils have been shown to be deeply involved in the process of metabolic diseases in both human and animal models: they can directly disrupt metabolic homeostasis by releasing inflammatory factors and regulate the balance of the immune-metabolic axis through phenotypic transformation. However, there are still significant gaps in the understanding of neutrophil transcriptional plasticity and subset heterogeneity, especially in the process of physiological-pathological state transition, the dynamic interaction mechanism between mitochondrial metabolic reprogramming and inflammasome activation still needs to be deeply revealed (Figure 4).
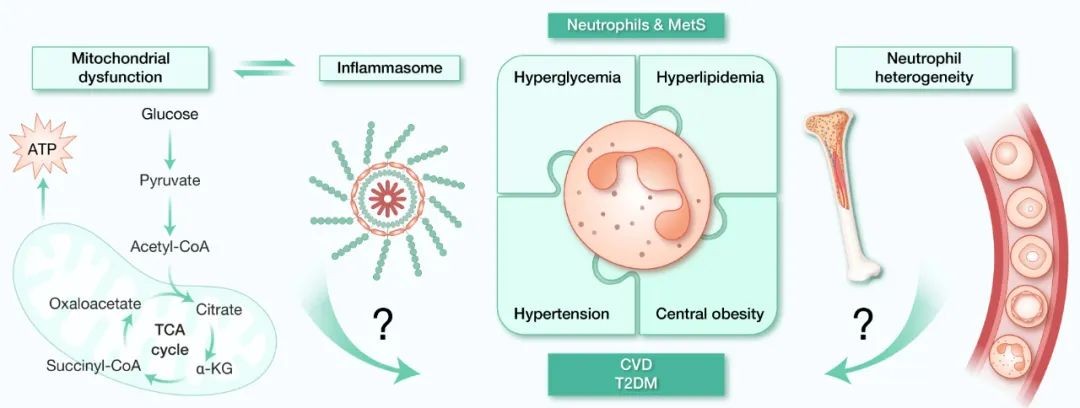
Fig. 4 Neutrophil heterogeneity-inflammasome-mitochondria: the regulatory axis of metabolic syndrome
Future research is expected to break through the traditional single-dimensional analysis framework, construct neutrophil functional maps with the help of multi-omics integration technology, and reveal their heterogeneous characteristics under the regulation of mitochondria-inflammasome axis. This will not only help to interpret the heterogeneous disease spectrum of metabolic syndrome, but also screen biomarkers with diagnostic value, providing new targets for precise prevention and individualized treatment of metabolic diseases such as T2DM and CVD. For example, intervention strategies targeting key nodes in neutrophil metabolism reprogramming, or reshaping metabolic-immune interaction networks can block the pathological cascade of metabolic syndrome from the root.
*This WeChat manuscript is a translated version, if there is any ambiguity, please refer to the original English version.

Hui Ping Yaw Postdoctoral First author
Institution: Shanghai Immune Therapy Institute Research direction: Exploration of the heterogeneity of neutrophils in health and disease

Lai Guan Ng Senior researcher Corresponding author
Institution: Shanghai Immune Therapy Institute Research interests: Intrinsic immunity, including the function and mechanism of myeloid cells in steady state and pathological state Citation format:Yaw HP, Devi S, Ng LG. Neutrophils: Key players in the metabolic syndrome puzzle. hLife 2025; 3: 121–131.
|