
Shanghai Immune Therapy Institute On January 14, 2025, Lai Guan Ng, a senior researcher at our institute, together with Daniela Cerezo-Wallis and Andres Hidalgo, professors at Yale University School of Medicine, and Melissa Ng, a researcher at the Singapore Science and Technology Research Authority (A*STAR), published a review titled "Adaptations of neutrophils in cancer" online in Immunity.This review provides an in-depth analysis of the adaptation mechanisms of neutrophils in the cancer environment and, based on these findings, explores effective strategies for cancer treatment. 
01 Research presentation Recently, a major theoretical breakthrough has revealed the plasticity of neutrophils in cancer, suggesting that they can be reprogrammed to promote the survival and proliferation of other cells1. This has also inspired researchers to explore and understand the adaptive capabilities of neutrophils in the cancer process and the molecular mechanisms that drive this adaptation process.
Still, several key questions remain open: Is this adaptation reprogramming mature neutrophils in the tumor microenvironment, or is neutrophils remodeling within the bone marrow? How do neutrophils play a dual role in tumor development to promote or inhibit tumor development? How do different tumor types induce multiple functions of neutrophils? This review not only comprehensively analyzes the above questions, but also explores effective cancer treatment strategies by elucidating the resilience of neutrophils in cancer.
1. Adaptation of neutrophils to homeostasis and cancer Neutrophils are an important part of the innate immune system. They are produced in the bone marrow and migrate in large quantities and rapidly to the damaged site of tissue once pathogen invasion is detected. Neutrophils engulf pathogens into the cell and degrade them through phagocytosis, while they attack foreign pathogens by releasing particulate matter and forming neutrophil extracellular trapping nets (NETs).
Although these typical antimicrobial functions have been valued over the past few decades, recent studies have found a wide range of phenotypic and functional heterogeneity in neutrophils in steady-state and under cancer. In the homeostasis of the body, neutrophils enter the tissue and their transcriptome changes. For example, it is regulated by prostaglandin E2 (PGE2) in lung tissue. Finally, endogenous signaling in tissues induces neutrophils to rapidly form the final phenotype. In tumor progression, immature neutrophils are released into the blood circulation in advance, and the tumor microenvironment specifically drives neutrophils to show different phenotypes. For example, in the hypoxic-glycolytic tumor microenvironment, immature and mature neutrophils undergo adaptive alterations, resulting in a phenotype that promotes angiogenesis. On the other hand, under the influence of leucine and other chemokines, mature neutrophils may be transformed into a phenotype with antigen presentation function.
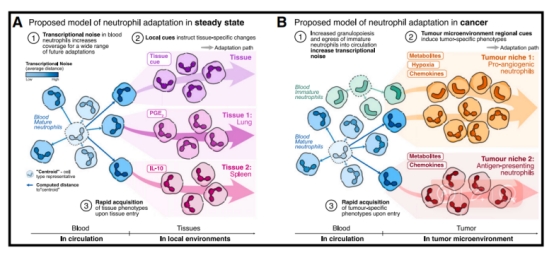
2. Systemic and local regulatory factors of neutrophils under cancer In the tumor microenvironment, neutrophils have a pro-angiogenesis and tumor growth phenotype. These phenotypes include the production of reactive oxygen species (ROS), the release of neutrophil elastase, and the increase in neutrophil extracellular trapping nets (NETs). Together, these changes promote the proliferation and migration of cancer cells and endothelial cells, thereby accelerating tumor growth and metastasis.
Studies have shown that in cancer, the homeostatic balance of circadian-night-mediated neutrophil release and aging is disrupted by tumor-derived cytokines such as CXCL2 and angiotensin II, while inhibition of CXCR2 or angiotensin II significantly reduces the metastasis of tumor cells to the lungs and liver. However, although studies have revealed that tumors are able to extend neutrophil lifespan, it is uncertain whether this phenotype will confer the ability of neutrophils to reprogram to promote tumor phenotypes more efficiently or quickly.
In addition, gender is also one of the key factors in the regulation of neutrophils. Little is known about how sex hormones regulate neutrophil infiltration in tumors and the mechanisms by which they adapt.
3. Engineered neutrophil-targeted cancer therapy Currently, a popular cancer treatment strategy is to use the tumor-oriented properties of neutrophils to deliver drugs. For example, neutrophils loaded with liposomal containing paclitaxel can effectively inhibit the recurrence of gliomas in mice by targeting residual tumor cells after surgical removal. At the same time, neutrophil membranes or extracellular vesicles (EVs) are also used to coat drug-loaded nanoparticles to improve their biocompatibility, stability, and targeting capabilities. Interestingly, this method enhances the ability of nanomaterials to cross the vascular endothelium, showing long-term effective therapeutic effects in cancer treatment. The researchers genetically engineered human pluripotent stem cells (hPSCs) through CRISPR-Cas9-mediated gene knock-in to express various anti-glioblastoma CAR structures with neutrophil-specific γ signaling domains, thereby constructing CAR-neutrophils with the best anti-tumor activity, which can specifically and non-invasively deliver and release nanodrugs that respond to the tumor microenvironment to target glioblastoma. without inducing additional inflammation at the tumor site. 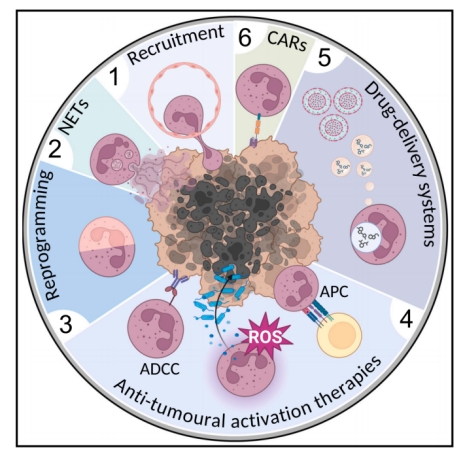
Neutrophils have only recently attracted widespread attention in the field of tumor immunology, and this breakthrough is mainly due to the rapid development of single-cell sequencing technology platforms and bioinformatics. As technology continues to innovate, clinical researchers are expected to leverage the high plasticity of neutrophils in cancer and other diseases to drive significant changes in clinical cancer treatment. 02 Original link https://doi.org/10.1016/j.immuni.2024.12.009 03 References 1. Deterministic reprogramming of neutrophils within tumors. Science. 2024 Jan 12;383(6679):eadf6493. doi: 10.1126/science.adf6493. Epub 2024 Jan 12. PMID: 38207030.
|